Alfa Cytology's huPBMC-T mice is an innovative platform specifically designed to accelerate T-cell immunotherapy development. By reconstituting a functional human T-cell immune system, this model provides drug developers with a highly reliable preclinical evaluation tool. Whether for CAR-T cell therapy, immune checkpoint inhibitors, or bispecific antibodies, the huPBMC-T model can provide highly clinically predictive efficacy data in a short period. We not only provide high-quality animal models but also offer end-to-end professional services from experimental design and model establishment to data analysis.
The huPBMC-T model establishes a robust humanized immune system by engrafting healthy donor-derived PBMCs into immunodeficient NCG or NSG mice, leading to a predominant reconstruction of functional human T cells.
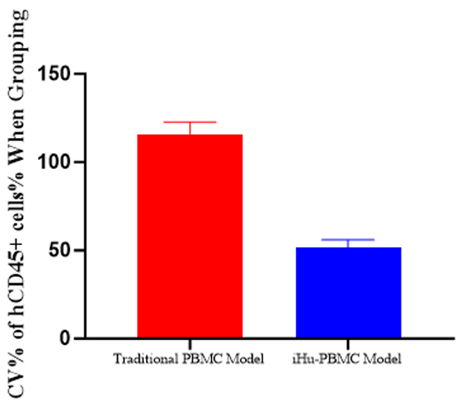 Fig.1 Compared to the traditional model, the HuPBMC-T model significantly improves the success rate and stability of model construction.
Fig.1 Compared to the traditional model, the HuPBMC-T model significantly improves the success rate and stability of model construction.
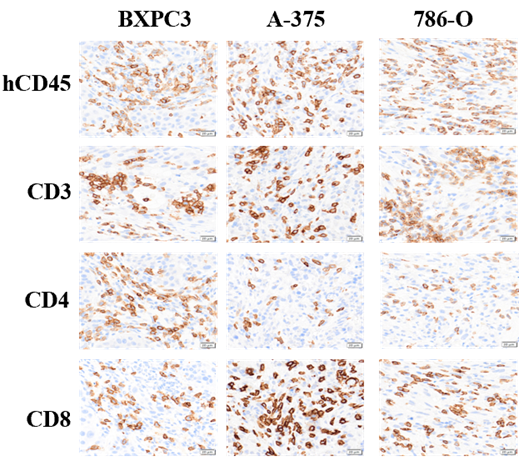 Fig.2 Significant human immune cell infiltration in the humanized immune system of xenografted tumors.
Fig.2 Significant human immune cell infiltration in the humanized immune system of xenografted tumors.
Excellent Model Stability
Modeling success rate up to 90%, significantly better than the 35-50% success rate of conventional models.
Adequate Experimental Window
Guarantees a stable experimental period of over 3 weeks, supporting complete pharmacodynamic evaluation.
Excellent Data Consistency
hCD45+ cell coefficient of variation controlled below 50%, ensuring reliable and reproducible experimental results.
Broad Tumor Model Compatibility
Validated for use with over 400 CDX models and over 400 PDX models.

Safety and efficacy evaluation of cellular therapies including CAR-T, TCR-T, and TIL.
Monotherapy or combination therapy research on checkpoint inhibitors including PD-1/PD-L1, CTLA-4, and 4-1BB.
Drug development and mechanism research on tumor microenvironment targets including TGF-β and Treg.
Overview
Assess antitumor activity and immune reconstitution in huPBMC-T humanized NCG model using a Treg-modulating anti-CD25 antibody and a cytokine regimen.
Study Information
Results
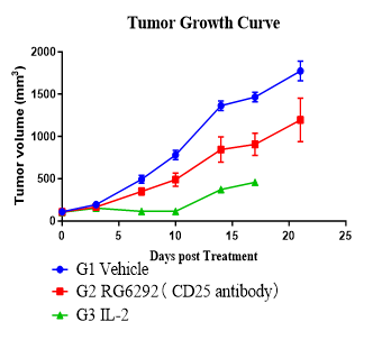
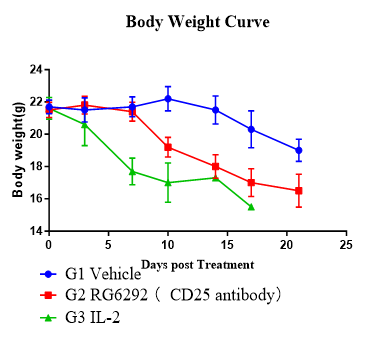
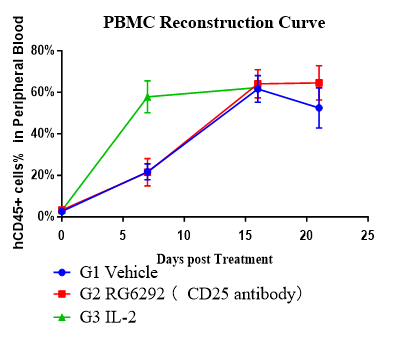
Fig. 3 Anti-tumor efficacy and human immune reconstitution in huPBMC-T humanized NCG model.
Overview
This study aimed to assess the efficacy of a PD-1 inhibitor, both as monotherapy and in combination with a TGF-β signaling inhibitor, showcasing the model's power in evaluating combination immunotherapy strategies.
Study Information
Results
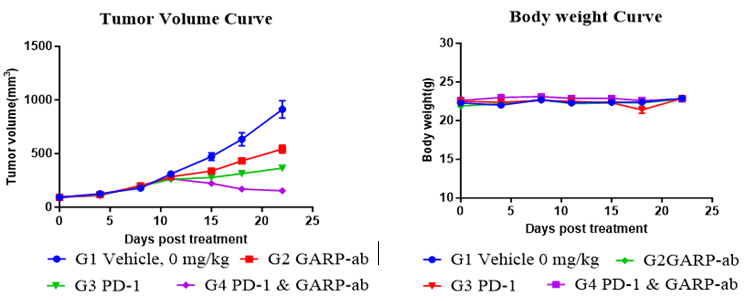 Fig. 4 Anti-tumor efficacy of PD-1, GARP-ab, and their combination in huPBMC-T humanized NCG model.
Fig. 4 Anti-tumor efficacy of PD-1, GARP-ab, and their combination in huPBMC-T humanized NCG model.
The huPBMC-T model is the ideal preclinical evaluation platform for your T-cell immunotherapy development. Alfa Cytology's Hu-Immune™ platform provides end-to-end professional services from model establishment to data analysis. Contact our scientific team now for customized solutions.
For research use only.