Multi-immune cell reconstitution
- Reconstitutes T, B, NK, DC and monocytes etc.
The huPBMC humanized model is a pivotal preclinical research tool. It involves the engraftment of human PBMCs into severely immunodeficient mice, leading to the reconstitution of a functional, T-cell-dominated human immune system. This model enables the evaluation of human immune cell responses to tumors or therapeutic agents in a live environment. Leveraging this established technology, Alfa Cytology's huPBMC humanized mouse models are the ideal tool for short-term, highly efficient immunotherapy screening. Through our optimized protocols, we achieve rapid and stable immune reconstitution, delivering powerful and reliable in vivo efficacy data within a compact timeline to accelerate your journey from candidate screening to mechanism verification.
Renowned for its short cycle and potent effects, the huPBMC humanized model is the "gold standard" in preclinical evaluation of oncology immunotherapies. It efficiently reconstructs functional human T cells and enables their infiltration into tumor tissue, closely mimicking human immune responses. This model demonstrates unparalleled sensitivity in evaluating T cell-mediated therapies like CAR-T and immune checkpoint inhibitors. Through stringent donor screening and quality control, we significantly enhance model success rate and stability, extending the experimental window to over 3 weeks to meet most efficacy study requirements.
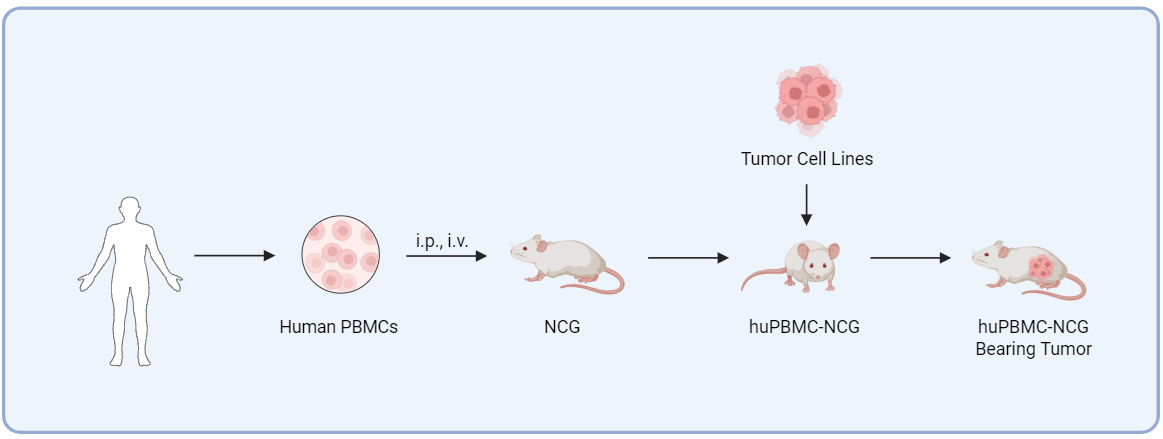 Fig.1 Schematic diagram of the huPBMC-NCG model establishment process.
Fig.1 Schematic diagram of the huPBMC-NCG model establishment process.
Both preclinical studies and published literature have validated the huPBMC humanized model as a robust platform for evaluating the efficacy of diverse cancer immunotherapies. As illustrated in the table below, this model has been successfully applied to the study of various solid tumors and hematological malignancies, including pancreatic cancer, ovarian cancer, lymphoma, and renal cell carcinoma. It effectively supports the assessment of core therapeutic strategies such as CAR-T cell therapy and immune checkpoint inhibitors. These cases underscore the critical value of the huPBMC model in mimicking human immune responses and predicting clinical efficacy.
| Cancers | Human Immune Cells | Mouse Strains | Immunotherapeutic Methods |
|---|---|---|---|
| Pancreatic Cancer | PBMC | NSG | CAR-T |
| Ovarian Cancer | PBMC | \ | CAR-T |
| Lymphoma | PBMC | NSG | Immune Checkpoint Inhibitor (anti-CTLA-4) |
| Renal Cell Carcinoma | PBMC | NSG | CAR-T / PD-L1 |
| Mesothelioma | PBMC | NOD | CAR-T / PD-1 |
| Colon/Gastric Cancer | PBMC | NSG | hCD137 / PD-1 |
| Non-small Cell Lung Cancer | PBMC | NSI | Immune Checkpoint Inhibitor (anti-D-1/PD-L1 antibody) |
Unlock the full potential of your immuno-oncology research with the Hu-Immune™ platform. Alfa Cytology provides a rapid, stable, and reliable huPBMC humanized mouse model generation service. Our proprietary Hu-Immune™ platform is designed to deliver robust human immune system reconstitution, accelerating your preclinical immunotherapy evaluation with highly predictive in vivo data.
| huPBMC Models | Key Features | Main Applications |
|---|---|---|
| huPBMC-T Mice | More stable T-cell reconstitution. Optimized for high-efficiency, stable T-cell reconstitution, making it the gold standard model for T cell-related immunotherapy research. |
CAR-T/TCR-T cell therapy evaluation, immune checkpoint inhibitor (e.g., PD-1/PD-L1) efficacy, bispecific antibody assessment. |
| huPBMC-NK/B Mice | Clinical-level NK or B cell reconstitution. Achieves NK and B cell reconstitution close to human physiological levels, ideal for evaluating NK/B cell-related functions. |
NK cell therapy, antibody-dependent cell-mediated cytotoxicity (ADCC) studies, B cell-targeting drug evaluation. |
| huPBMC-Mono Mice | Macrophage and DC reconstitution for cancer vaccines. Focuses on the reconstitution and function of monocytes/macrophages/dendritic cells for studying the role of myeloid cells in the tumor microenvironment. |
Macrophage-targeting drugs (e.g., CD47 antibodies), cancer vaccine evaluation, tumor microenvironment research. |
| huPBMC-MHC KO Mice | Longer dosing window for in vivo immune therapy efficacy. Significantly delays graft-versus-host disease (GvHD) onset through innovative technology, effectively extending the treatment window for long-term dosing studies. |
Long-term efficacy studies, combination therapy evaluation, exploratory research requiring wider observation windows. |
Alfa Cytology has established a standardized workflow for huPBMC model studies, ensuring exceptional reproducibility and data reliability at every stage, from model generation to final data delivery.

Donor Screening & PBMC Preparation
High-quality human PBMCs are isolated from pre-screened, healthy donors to guarantee reliable sources and stable cell viability, laying the foundation for successful model establishment.

Immune System Humanization & Reconstitution
PBMCs are engrafted into immunodeficient mice. Through precise process management and regular monitoring, stable and efficient, T-cell-dominated immune reconstitution is achieved.

Tumor Model Establishment
Following successful immune reconstitution, specific human tumor cell lines or patient-derived tumor tissues are implanted to establish stable tumor-bearing models for efficacy evaluation.

Drug Treatment & Efficacy Evaluation
Within a defined therapeutic window, test articles (such as immune checkpoint inhibitors, cell therapies, or bispecific antibodies) are administered, and their antitumor activity and immunomodulatory effects are systematically assessed.

Endpoint Analysis & Data Reporting
A comprehensive analysis is conducted, including evaluation of tumor growth inhibition, immune cell infiltration, cytokine secretion, and potential toxicity, culminating in a detailed and robust study report.
Overview
Evaluate antitumor activity of a CD3 bispecific antibody (BsAb) and confirm robust human immune reconstitution in a Hu-Immune™ huPBMC humanized NCG model.
Study Information
Results
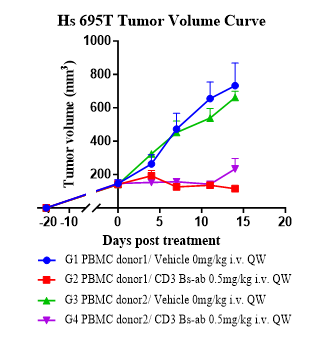
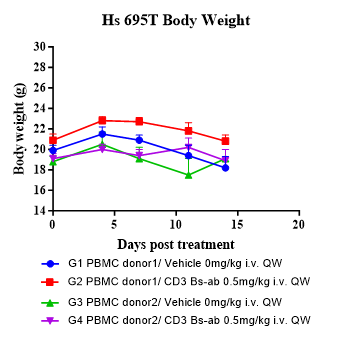
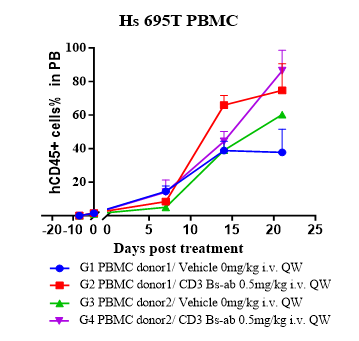
Fig.2 CD3 BsAb efficacy study in huPBMC Humanized Model.

Multi-immune cell reconstitution

Highly versatile, simple solution

Stable Experimental system

Low Experimental Cost
Ready to obtain fast and reliable in vivo immune efficacy data? Alfa Cytology's huPBMC humanized models are designed for efficient and precise researchers like you. Contact our scientific team today for a customized study proposal.
For research use only.