Alfa Cytology's huPBMC-MHC KO mice represent a significant advancement in humanized model technology, specifically engineered to address the challenge of graft-versus-host disease (GvHD). By utilizing major histocompatibility complex (MHC) knockout (KO) human PBMCs for engraftment, this model effectively delays the onset of GvHD, thereby extending the critical experimental window for in vivo efficacy studies. It is an ideal platform for researchers requiring longer-term observation, combination therapy evaluation, and exploratory studies that exceed the timeframe of conventional huPBMC models.
The huPBMC-MHC KO model is established by engrafting immunodeficient NCG or NSG mice with healthy donor-derived PBMCs in which major histocompatibility complex (MHC) molecules have been knocked out. This strategic modification significantly reduces the recognition of host mouse tissues by human T cells, the primary drivers of GvHD. While retaining the rapid setup and robust human immune cell reconstitution of the standard huPBMC model, the huPBMC-MHC KO variant provides a substantially extended treatment window. This allows for more complex study designs, including long-term dosing regimens, detailed pharmacodynamic assessments, and the evaluation of delayed immune responses.

Extended Experimental Window
Significantly delays GvHD onset, providing a longer stable period for efficacy observation and repeated dosing.

Retained Immune Functionality
Maintains the rapid reconstruction and functional activity of key human immune cells, such as T cells, while mitigating GvHD.

Ideal for Complex Study Designs
Supports long-term efficacy studies, combination therapy evaluation, and exploratory research requiring prolonged observation.

Technologically Advanced Platform
Leverages cutting-edge MHC knockout technology to overcome a key limitation of traditional humanized models.
Long-Term Immunotherapy Efficacy Studies
Evaluation of sustained efficacy and durability of response for cell therapies, bispecific antibodies, and other immunomodulatory agents.
Combination Therapy Regimen Evaluation
Assessment of complex combination strategies that require an extended timeline to observe synergistic effects or delayed responses.
Exploratory & Mechanistic Research
In-depth investigation of long-term immune cell persistence, memory formation, and tumor-immune ecosystem evolution.
Overview
Directly compare in vivo tolerability, survival, and study window between a second-generation huPBMC–MHC-KO model and a traditional PBMC humanized model under a fixed therapeutic dosing regimen.
Study Information
Results
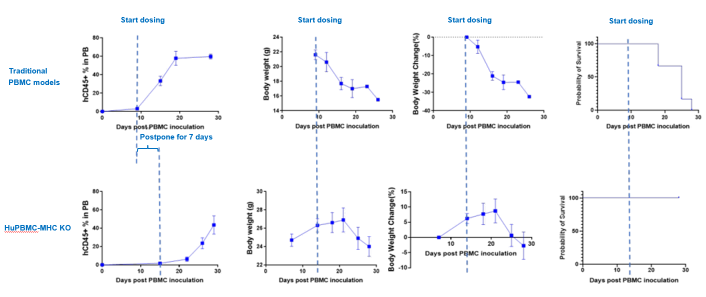 Fig.1 Tolerability and survival comparison between traditional PBMC humanized and second-generation huPBMC–MHC-KO models.
Fig.1 Tolerability and survival comparison between traditional PBMC humanized and second-generation huPBMC–MHC-KO models.
The huPBMC-MHC KO model is the definitive solution for preclinical studies that demand a longer and more stable experimental window. Alfa Cytology's Hu-Immune™ platform combines this advanced model with end-to-end services to empower your long-term immunotherapy research. Contact our scientific team now for customized solutions.
For research use only.