Alfa Cytology's huPBMC-NK mice are an advanced preclinical platform specifically engineered for the evaluation of NK cell-mediated immunotherapies. By achieving physiological-level reconstitution of functional human NK cells, this model provides a powerful and predictive tool for assessing antibody-dependent cellular cytotoxicity (ADCC), NK cell engagers, CAR-NK cell therapies, and immunostimulatory agents. We offer comprehensive, end-to-end services from model establishment to robust functional analysis, accelerating your NK-cell-focused drug development programs.
Introduction to huPBMC-NK Mice
The huPBMC-NK model is established by engrafting healthy donor-derived PBMCs into immunodeficient NCG mice, utilizing proprietary protocols optimized for robust human NK cell reconstitution over other immune cell types.
- Rapid, High-Level Reconstitution: Hu-Immune™ platform achieves human NK cell concentrations of up to 105/mL in peripheral blood, closely mirroring human physiological levels.
- Physiological NK Phenotype: The reconstructed NK cell population maintains a CD56+CD16+ to CD56+CD16- ratio of approximately 90:10, matching the functional subset distribution found in human peripheral blood.
- Extended Experimental Window: The model maintains stable NK cell reconstitution for 4-6 weeks, supporting complete efficacy studies for a wide range of therapeutic modalities.
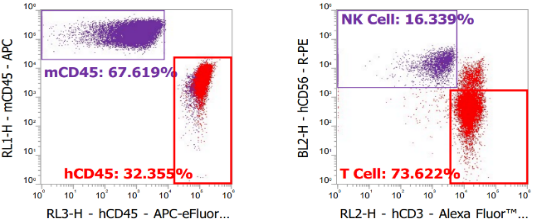 Fig.1 huPBMC-NK: high-level NK reconstruction.
Fig.1 huPBMC-NK: high-level NK reconstruction.
Advantages of huPBMC-NK Mice
Highly versatile human NK reconstitution
- Compatible with 400+ CDX models and 400+ PDX models.
Clinical-level NK reconstitution
- Innovative methods can reconstitute 105/mL NK cells in mouse peripheral blood, similar to clinical level.
Keep normal function of NK cells
- The reconstructed NK cells are mainly CD16+ cells, which are closer to the situation in human peripheral blood.
Applications of huPBMC-NK Mice
Critical assessment of therapeutic antibodies reliant on NK cell-mediated cytotoxicity.
- NK Cell Engager Assessment
Evaluation of bispecific and multispecific molecules designed to activate and redirect NK cells.
- CAR-NK Cell Therapy Testing
In vivo validation of CAR-NK cell persistence, trafficking, and antitumor efficacy.
- NK Cell Checkpoint Studies
Investigation of next-generation immune checkpoints such as NKG2A.
- Immunostimulatory Cytokine Evaluation
Assessment of cytokines like IL-15 for their ability to expand and activate NK cells.
Case Study
Case 1: Evaluation of Superior ADCC Drug Evaluation in huPBMC-NK Model
Overview
Assess the antitumor activity of an ADCC-dependent antibody (Imab362) in a huPBMC-NK humanized NCG model and benchmark against CD34⁺ HSC–humanized and SCID models.
Study Information
- Humanized Model: huPBMC-NK on NCG strain, huPBMC-HSC mice and SCID mice (mouse-derived NK cells)
- Tumor Model: CLDN18.2-positive gastric cancer CDX
- Treatment: Imab362 (CLDN18.2-targeting mAb)
Results
- Enhanced Predictive Value: The huPBMC-NK model demonstrated significantly superior tumor growth inhibition compared to both SCID (with mouse NK cells) and CD34⁺ HSC humanized models.
- Model Relevance: Validates the model as the preferred platform for assessing the efficacy of ADCC-dependent therapeutic antibodies.
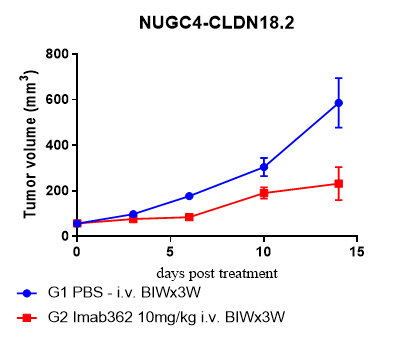 Innovative HuPBMC NK
Innovative HuPBMC NK
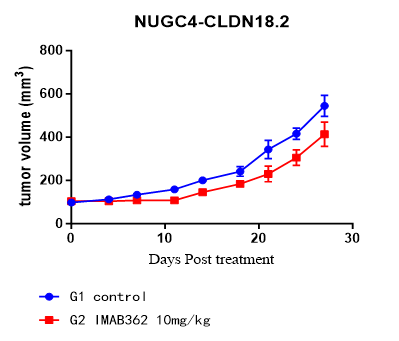 CD34+HSC humanized mouse model
CD34+HSC humanized mouse model
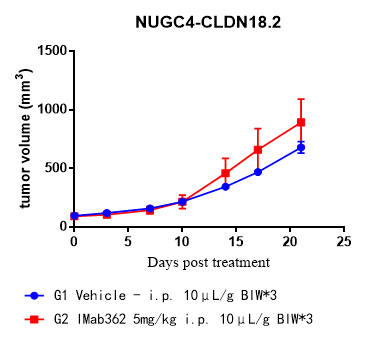 SCID mice (can provide mouse-derived NK cells)
SCID mice (can provide mouse-derived NK cells)
Fig.2 Comparative antitumor activity of an ADCC-dependent anti-CLDN18.2 antibody (Imab362) across preclinical models.
Case 2: NK-Cell Expansion and Anti-tumor Activity with hIL-15 in huPBMC-NK Humanized NCG Model
Overview
Assess pharmacodynamic expansion of human NK cells and antitumor activity following human IL-15 (hIL-15) stimulation in huPBMC-NK humanized NCG model, and include a CT26 syngeneic model as a comparator.
Study Information
- Humanized Model: huPBMC-NK on NCG strain
- Stimulant: Human IL-15
Results
- Potent NK Cell Expansion: NK cells in the huPBMC-NK model exhibited a poten expansion upon stimulation with hIL-15.
- Functional Response: The robust expansion fully demonstrates the platform's superior capability to evaluate the immunostimulatory effects of cytokines and other activating agents.
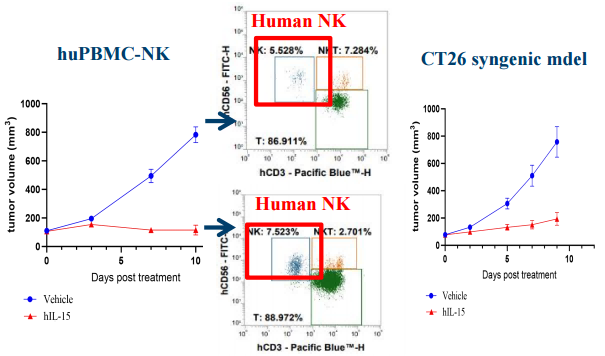 Fig.3 Robust expansion of human NK cells in response to cytokine stimulation.
Fig.3 Robust expansion of human NK cells in response to cytokine stimulation.
Case 3: Anti-tumor Activity of an Anti-NKG2A Antibody in a huPBMC-NK Humanized NCG Model
Overview
Evaluate antitumor activity of Monalizumab (anti-NKG2A) in a huPBMC-NK humanized NCG model and illustrate the mechanism by which NKG2A blockade can enhance human NK-cell function.
Study Information
- Humanized Model: huPBMC-NK on NCG strain
- Tumor Model: Relevant tumor CDX
- Treatment: Anti-NKG2A therapeutic antibody
Results
- Enhanced NK-cell Activity: Treatment with the NKG2A inhibitor demonstrated significant enhancement of NK cell-mediated antitumor activity.
- Target Validation: The study provided compelling in vivo evidence for NKG2A as a therapeutic target and validated the model's suitability for evaluating next-generation NK cell checkpoint immunotherapies.
- Potent Tumor Growth Inhibition: The enhanced NK cell activity led to potent tumor growth inhibition.
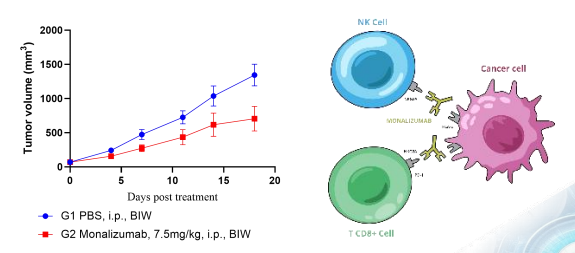 Fig.4 Enhanced anti-tumor efficacy via NK cell checkpoint blockade.
Fig.4 Enhanced anti-tumor efficacy via NK cell checkpoint blockade.
The huPBMC-NK model is the definitive preclinical solution for your NK cell immunotherapy discovery. Alfa Cytology's Hu-Immune™ platform delivers end-to-end services—from optimized model establishment to robust functional analysis. Contact our scientific team now for customized solutions.
For research use only.
Related Services
 Fig.1 huPBMC-NK: high-level NK reconstruction.
Fig.1 huPBMC-NK: high-level NK reconstruction. Innovative HuPBMC NK
Innovative HuPBMC NK CD34+HSC humanized mouse model
CD34+HSC humanized mouse model SCID mice (can provide mouse-derived NK cells)
SCID mice (can provide mouse-derived NK cells) Fig.3 Robust expansion of human NK cells in response to cytokine stimulation.
Fig.3 Robust expansion of human NK cells in response to cytokine stimulation. Fig.4 Enhanced anti-tumor efficacy via NK cell checkpoint blockade.
Fig.4 Enhanced anti-tumor efficacy via NK cell checkpoint blockade.